Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Micheau, C.; Ueda, Yuki; Motokawa, Ryuhei; Akutsu, Kazuhiro*; Yamada, Norifumi*; Yamada, Masako*; Moussaoui, S. A.*; Makombe, E.*; Meyer, D.*; Berthon, L.*; et al.
Journal of Molecular Liquids, 401, p.124372_1 - 124372_12, 2024/05
Supramolecular organization of extractant molecules impacts metal ions separation behavior. Probing bulk and interfacial structures of the relevant systems is expected to provide key insights into the metal ion selectivity and kinetic aspects. The supramolecular features of two solvent extraction systems based on malonamide extractants THMA in toluene and DBMA in n-heptane were studied using small-angle X-ray scattering for the organic bulk phases, as well as interfacial tension and neutron reflectivity measurements for the interfaces. In the bulk solution, THMA forms dimeric/trimeric associates but no aggregates in toluene, while DBMA forms large aggregates in n-heptane. On the other hand, THMA accumulates in a diffuse layer at the interface at high THMA concentration, whereas DBMA forms a compact but thinner layer. After Pd(II) extraction, the thickness of interfacial layers decreases in the case of THMA, and totally vanishes in the case of DBMA. Based on these new structural information, two mechanisms are proposed for Pd(II) and Nd(III) extraction with malonamides. In toluene, THMA associates slightly accumulate in the vicinity of the interface, then coordinate Pd(II) and diffuse into the organic bulk phase. In n-heptane, DBMA aggregates adsorb at the interface then pick up Nd(III) cations in their polar cores and finally diffuse into the bulk.
 -Ga
-Ga O
O (001)
(001)Tsai, Y. H.*; Kobata, Masaaki; Fukuda, Tatsuo; Tanida, Hajime; Kobayashi, Toru; Yamashita, Yoshiyuki*
Applied Physics Letters, 124(11), p.112105_1 - 112105_5, 2024/03
 Pt
Pt Al
Al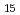
Ota, Kyugo*; Watabe, Yuki*; Haga, Yoshinori; Iesari, F.*; Okajima, Toshihiko*; Matsumoto, Yuji*
Symmetry (Internet), 15(8), p.1488_1 - 1488_13, 2023/07
Times Cited Count:1 Percentile:66.09(Multidisciplinary Sciences) X-ray synchrotron diffraction
X-ray synchrotron diffractionLi, H.*; Liu, Y.*; Zhao, W.*; Liu, B.*; Tominaga, Aki; Shobu, Takahisa; Wei, D.*
International Journal of Plasticity, 165, p.103612_1 - 103612_20, 2023/06
Times Cited Count:2 Percentile:78.7(Engineering, Mechanical)In order to clarify the strength properties of Co-free maraging steel,  tensile experiment using high energy synchrotron X-ray diffraction was performed. Diffraction profiles from the martensitic and austenitic phases were obtained, and their strength and width were observed to vary as loading. Analysis of the diffraction profiles showed that the content of martensite in the as-aged material decreased slowly at low stress levels and decreased rapidly at high stress levels. On the other hand, the austenite phase in the as-solution materials was significantly transformed the martensite phase as the stress increased. It was clarified to be responsible for their respective strength properties.
tensile experiment using high energy synchrotron X-ray diffraction was performed. Diffraction profiles from the martensitic and austenitic phases were obtained, and their strength and width were observed to vary as loading. Analysis of the diffraction profiles showed that the content of martensite in the as-aged material decreased slowly at low stress levels and decreased rapidly at high stress levels. On the other hand, the austenite phase in the as-solution materials was significantly transformed the martensite phase as the stress increased. It was clarified to be responsible for their respective strength properties.
Tsai, T.-H.; Sasaki, Shinji; Maeda, Koji
Journal of Nuclear Science and Technology, 60(6), p.715 - 723, 2023/06
Times Cited Count:1 Percentile:31.61(Nuclear Science & Technology)Yamazaki, Yasuhiro*; Shinomiya, Keisuke*; Okumura, Tadaharu*; Suzuki, Kenji*; Shobu, Takahisa; Nakamura, Yuiga*
Quantum Beam Science (Internet), 7(2), p.14_1 - 14_12, 2023/05
Yoshida, Yukihiko
IL Nuovo Cimento, 46(2), p.33_1 - 33_8, 2023/03
Suzuki, Tomoya*; Otsubo, Ukyo*; Ogata, Takeshi*; Shiwaku, Hideaki; Kobayashi, Toru; Yaita, Tsuyoshi; Matsuoka, Mitsuaki*; Murayama, Norihiro*; Narita, Hirokazu*
Separation and Purification Technology, 308, p.122943_1 - 122943_7, 2023/03
Times Cited Count:2 Percentile:24.43(Engineering, Chemical)HNO leaching is used in recycling Pd metal from spent products that primarily contain Ag, and most Pd residues are separated from solutions containing Ag(I). However, a small amount of Pd(II) often remains in these Ag(I) solutions. Therefore, the separation of Pd(II) and Ag(I) in HNO
leaching is used in recycling Pd metal from spent products that primarily contain Ag, and most Pd residues are separated from solutions containing Ag(I). However, a small amount of Pd(II) often remains in these Ag(I) solutions. Therefore, the separation of Pd(II) and Ag(I) in HNO solutions is essential to promote efficient Pd recycling. In this study, the separation of Pd(II) and Ag(I) in HNO
solutions is essential to promote efficient Pd recycling. In this study, the separation of Pd(II) and Ag(I) in HNO solutions was investigated using four N-donor-type adsorbents functionalized with amine (R-Amine), iminodiacetic acid (R-IDA), pyridine (R-Py), or bis-picolylamine (R-BPA). R-Amine, R-IDA, and R-Py selectively adsorbed Pd(II) over Ag(I), Cu(II), Ni(II), and Fe(III) from HNO
solutions was investigated using four N-donor-type adsorbents functionalized with amine (R-Amine), iminodiacetic acid (R-IDA), pyridine (R-Py), or bis-picolylamine (R-BPA). R-Amine, R-IDA, and R-Py selectively adsorbed Pd(II) over Ag(I), Cu(II), Ni(II), and Fe(III) from HNO solutions (0.3-7 M), but R-Amine exhibited a lower Pd adsorption efficiency. In contrast,
solutions (0.3-7 M), but R-Amine exhibited a lower Pd adsorption efficiency. In contrast,  90% of Pd(II), Ag(I), and Cu(II) were adsorbed by R-BPA over the entire range of HNO
90% of Pd(II), Ag(I), and Cu(II) were adsorbed by R-BPA over the entire range of HNO concentrations. Structural analyses of the adsorbed metal ions using Fourier transform infrared spectroscopy and extended X-ray absorption fine structure spectroscopy revealed the separation mechanisms of the N-donor-type adsorbents. Pd(II) adsorption on R-IDA, R-Py, and R-BPA occurred via Pd(II) coordination of the functional groups (iminodiacetic acid, pyridine, and bis-picolylamine, respectively), whereas that on R-Amine occurred via anion exchange of NO
concentrations. Structural analyses of the adsorbed metal ions using Fourier transform infrared spectroscopy and extended X-ray absorption fine structure spectroscopy revealed the separation mechanisms of the N-donor-type adsorbents. Pd(II) adsorption on R-IDA, R-Py, and R-BPA occurred via Pd(II) coordination of the functional groups (iminodiacetic acid, pyridine, and bis-picolylamine, respectively), whereas that on R-Amine occurred via anion exchange of NO
 with [Pd(NO
with [Pd(NO )
) ]
] . The coordinative adsorption mechanisms resulted in the higher Pd(II) adsorption behaviors of R-IDA, R-Py, and R-BPA. HCl (5.0 M) and thiourea (0.1 M) eluents desorbed 83% of Pd(II) from R-IDA and 95% from R-Py, respectively. R-Py was the most effective Pd(II) adsorbent based on adsorption selectivity and desorption efficiency.
. The coordinative adsorption mechanisms resulted in the higher Pd(II) adsorption behaviors of R-IDA, R-Py, and R-BPA. HCl (5.0 M) and thiourea (0.1 M) eluents desorbed 83% of Pd(II) from R-IDA and 95% from R-Py, respectively. R-Py was the most effective Pd(II) adsorbent based on adsorption selectivity and desorption efficiency.
 synchrotron X-ray scattering and nanoindentation test
synchrotron X-ray scattering and nanoindentation testIm, S.*; Jee, H.*; Suh, H.*; Kanematsu, Manabu*; Morooka, Satoshi; Choe, H.*; Nishio, Yuhei*; Machida, Akihiko*; Kim, J.*; Lim, S.*; et al.
Construction and Building Materials, 365, p.130034_1 - 130034_18, 2023/02
Times Cited Count:6 Percentile:70.19(Construction & Building Technology)Wang, Q.*; Hu, Q.*; Zhao, C.*; Yang, X.*; Zhang, T.*; Ilavsky, J.*; Kuzmenko, I.*; Ma, B.*; Tachi, Yukio
International Journal of Coal Geology, 261, p.104093_1 - 104093_15, 2022/09
Times Cited Count:5 Percentile:69.58(Energy & Fuels) and CrUO
and CrUO
Akiyama, Daisuke*; Kusaka, Ryoji; Kumagai, Yuta; Nakada, Masami; Watanabe, Masayuki; Okamoto, Yoshihiro; Nagai, Takayuki; Sato, Nobuaki*; Kirishima, Akira*
Journal of Nuclear Materials, 568, p.153847_1 - 153847_10, 2022/09
Times Cited Count:3 Percentile:68.71(Materials Science, Multidisciplinary)FeUO , CrUO
, CrUO , and Fe
, and Fe Cr
Cr UO
UO are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO
are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO and CrUO
and CrUO , their crystal structures were evaluated in detail. A Raman band was observed at 700 cm
, their crystal structures were evaluated in detail. A Raman band was observed at 700 cm in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe
in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe Cr
Cr UO
UO was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M
was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M ssbauer measurements indicated that the Fe in FeUO
ssbauer measurements indicated that the Fe in FeUO and Fe
and Fe Cr
Cr UO
UO were trivalent. Furthermore, Fe
were trivalent. Furthermore, Fe Cr
Cr UO
UO lost its symmetry around Fe
lost its symmetry around Fe with increasing electron densities around Fe
with increasing electron densities around Fe , as the abundance of Cr increased. These results suggested no significant structural differences between FeUO
, as the abundance of Cr increased. These results suggested no significant structural differences between FeUO and CrUO
and CrUO . Thermogravimetric measurements for UO
. Thermogravimetric measurements for UO , FeUO
, FeUO , and CrUO
, and CrUO showed that the temperature at which FeUO
showed that the temperature at which FeUO decomposed under an oxidizing condition (approximately 800
decomposed under an oxidizing condition (approximately 800  C) was significantly lower than the temperature at which the decomposition of CrUO
C) was significantly lower than the temperature at which the decomposition of CrUO started (approximately 1250
started (approximately 1250  C). Based on these results, we concluded that the decomposition of FeUO
C). Based on these results, we concluded that the decomposition of FeUO was triggered by an "in-crystal" redox reaction, i.e., Fe
was triggered by an "in-crystal" redox reaction, i.e., Fe
 U
U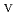
 Fe
Fe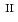
 U
U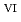 , which would not occur in the CrUO
, which would not occur in the CrUO lattice because Cr
lattice because Cr could never be reduced under the investigated condition. Finally, the existence of Cr
could never be reduced under the investigated condition. Finally, the existence of Cr in FexCr
in FexCr UO
UO effectively suppressed the decomposition of the Fe
effectively suppressed the decomposition of the Fe Cr
Cr UO
UO crystal, even at a very low Cr content.
crystal, even at a very low Cr content.
Im, S.*; Jee, H.*; Suh, H.*; Kanematsu, Manabu*; Morooka, Satoshi; Koyama, Taku*; Nishio, Yuhei*; Machida, Akihiko*; Kim, J.*; Bae, S.*
Journal of the American Ceramic Society, 104(9), p.4803 - 4818, 2021/09
Times Cited Count:18 Percentile:85.31(Materials Science, Ceramics)Grazzi, F.*; Cialdai, C.*; Manetti, M.*; Massi, M.*; Morigi, M. P.*; Bettuzzi, M.*; Brancaccio, R.*; Albertin, F.*; Shinohara, Takenao; Kai, Tetsuya; et al.
Rendiconti Lincei. Scienze Fisiche e Naturali, 32(3), p.463 - 477, 2021/09
Times Cited Count:3 Percentile:22.01(Multidisciplinary Sciences)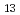 } polyoxo cluster U
} polyoxo cluster U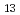 O
O Cl
Cl (MeO)
(MeO) (x = 2.3, MeO = methoxide)
(x = 2.3, MeO = methoxide)Fichter, S.*; Radoske, T.*; Ikeda, Atsushi
Acta Crystallographica Section E; Crystallographic Communications (Internet), 77(8), p.847 - 852, 2021/08
Inami, Toshiya*; Shobu, Takahisa; Ishii, Kenji*
IEEE Transactions on Magnetics, 57(3, Part 2), p.6400105_1 - 6400105_5, 2021/03
Times Cited Count:0 Percentile:0(Engineering, Electrical & Electronic)Abe, Yuta; Yamashita, Takuya; Sato, Ikken; Nakagiri, Toshio; Ishimi, Akihiro
Journal of Nuclear Engineering and Radiation Science, 6(2), p.021113_1 - 021113_9, 2020/04
Takahashi, Hiroaki*; Tachi, Yukio
Applied Clay Science, 168, p.211 - 222, 2019/02
Times Cited Count:9 Percentile:47.52(Chemistry, Physical)Microstructural and mass transport properties of compacted Na- and Cs-montmorillonites with different swelling properties were investigated by combining 3D microstructure analysis using nanofocus X-ray CT and diffusion measurement of HDO. The X-ray CT observations indicated that macropores in the dry state of compacted Na-montmorillonite are filled with gel phases, and the grain sizes of clay particles shifted toward smaller values through the saturation and swelling processes. By contrast, no gel phase and no decrease in the grain and pore volumes were observed for saturated Cs-montmorillonite. The geometrical factors of the macropores including tortuosity and geometric constrictivity of saturated Cs-montmorillonite determined by the X-ray CT was consistent with the corresponding values derived in the HDO diffusion test. In the case of Na-montmorillonite, the larger differences between the geometric factors evaluated by the X-ray CT and the diffusion tests can be explained by the electrostatic constrictivity factor and the additional geometrical factors in gel phase and interlayer that are smaller than the detection limit of the X-ray CT.
Nakamura, Keisuke; Morishita, Yuki; Takasaki, Koji; Maehata, Keisuke*; Sugimoto, Tetsuya*; Kiguchi, Yu*; Iyomoto, Naoko*; Mitsuda, Kazuhisa*
Journal of Low Temperature Physics, 193(3-4), p.314 - 320, 2018/11
Times Cited Count:0 Percentile:0(Physics, Applied)Yoshida, Koji*; Inoue, Takuya*; Torigoe, Motokatsu*; Yamada, Takeshi*; Shibata, Kaoru; Yamaguchi, Toshio*
Journal of Chemical Physics, 149(12), p.124502_1 - 124502_10, 2018/09
Times Cited Count:4 Percentile:17.83(Chemistry, Physical)Differential scanning calorimetry, X-ray diffraction, and quasi-elastic neutron scattering (QENS) measurements of aqueous glycine solutions confined in mesoporous silica (MCM-41) were performed at different glycine concentrations, pH, and loading ratio (= mass of glycine solution / mass of dry MCM-41) in the temperature range from 305 to 180 K to discuss the confinement effect on the thermal behavior, the structure, and the dynamic properties of the solutions.
Oka, Hiroshi; Tanno, Takashi; Otsuka, Satoshi; Yano, Yasuhide; Kaito, Takeji
Nuclear Materials and Energy (Internet), 16, p.230 - 237, 2018/08
Times Cited Count:4 Percentile:38.58(Nuclear Science & Technology)